OXC-201 introduction
Oxcia is a pioneer in the new and exciting research area that explores the role of oxidative DNA damage in inflammation and fibrosis and how this can be used in novel treatments. OXC-201 is a new type of DDR treatment that blocks inflammation and fibrosis by inhibiting an enzyme, OGG1. OXC-201 is in the preclinical phase with the potential to become a “first-in-class” treatment for idiopathic pulmonary fibrosis (IPF)
In inflammation and fibrosis, the tissue is exposed to oxidative stress, which causes oxidative damage to DNA. OGG1 is a protein that binds to these damaged bases in a process called base excision repair, which was discovered by Professor Tomas Lindahl, and for which he was awarded the Nobel Prize in 2015. Recent evidence highlights an additional function of OGG1, a role in cellular reprogramming in inflammatory and fibrotic diseases. With the development of OXC-201 in IPF, Oxcia is taking advantage of these processes.

Problem
Idiopathic Pulmonary Fibrosis (IPF) is a serious progressive lung disease that severely affects physical well-being and is characterized by a high degree of illness and mortality. IPF is chronic (long-term) and the condition develops when lung tissue becomes thick and stiff. Over time permanent scarring in the lung, called fibrosis, happens and this makes it difficult to breathe. Besides shortness of breath that becomes worse over time, additional symptoms can be a dry cough that is not improving, achy joints and muscles, feeling tired/weak and slowly losing weight without trying. Common complications of IPF are pulmonary hypertension and respiratory failure. This happens when the lungs cannot deliver enough oxygen to the rest of the body, including the brain.
The underlying cause of IPF is unknown, but risk factors are for instance male sex, aging, smoking, diabetes and a family history of IPF.
Today there is no cure for IPF. Average survival is only 3-5 years after diagnosis (Swedish Pulmonary Medicine Association 2019).There are two approved drugs on the market – pirfenidone and nintedanib. These treatments do not cure the disease but slow down the progression of IPF and help the lungs work better. They are limited by adverse effects and drug interactions.
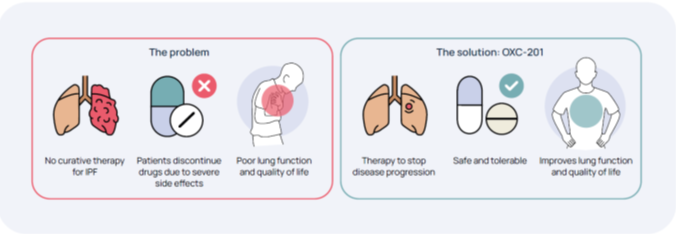
Solution – OXC-201 tackles IPF in a completely novel way
Oxcia is developing a novel solution, OXC-201, based on completely new science published in Science 2018 by Professor Helleday, academic collaborators and Oxcia team. OXC-201 has the potential to improve the lives of patients suffering from the currently incurable IPF. The goal of Oxcia’s research is to target the underlying inflammation and fibrosis in the IPF to stop disease progression, improve lung function and potentially halt the disease.
Mechanism of action
To understand how OXC-201 works, we first need to explain the underlying disease mechanisms of IPF. These involves a complex interplay between cell types and inflammatory and fibrotic signalling pathways. IPF starts with small injury-induced inflammation in the tiny air sacs located in lower part of the lung, also called lung alveoli, where the respiration occurs. With help from a multifunctional mediator (TGF-beta), tissue damage induces production of ROS and thus generates oxidative stress.
This in turn causes oxidative DNA damage in form of accumulation of 8-oxoG in DNA. The DNA repair enzyme OGG1 is the main protein in the cell to recognize oxidative (8-oxoG) damage. The binding of these two molecules, 8-oxoG and OGG1, plays an important role in inflammatory and fibrotic responses, leading to increase of inflammatory and fibrotic biomarkers, recruitment of immune cells and fibroblasts to the site of inflammation and accumulation of collagen in cellular walls in lung alveoli. In IPF these processes are chronic leading to formation of fibrotic scars, stiffness of the lung tissue and a progressive decrease in lung capacity. Interestingly, lungs from IPF patients have increased OGG1 expression than a healthy lung.
Oxcia’s novel approach, OXC-201 binds to OGG1 and inhibits the function of the enzyme. More precisely, by targeting OGG1, OXC-201 inhibits binding of OGG1 to 8-oxoG and thereby suppresses a broad range of pro-inflammatory and pro-fibrotic signalling. The end result is decreased lung collagen accumulation, reduced lung damage and maintained structure of the lung alveoli.
In summary, OXC-201 tackles several components involved in the pathological processes in IPF and thus has the potential to halt the disease progression and avoid tissue damage.
OGG1 plays a significant role in modulating inflammation and fibrogenesis which opens up opportunities in several indications. OXC-201 has the potential to revolutionize the market for anti-fibrotic agents and also has significant anti-inflammatory properties, effects already demonstrated in disease models for acute lung injury (ARDS) and allergic asthma.
Further research of OXC-201 is ongoing, investigating whether it is a suitable clinical drug candidate. Oxcia has received a grant from the European Innovation Council (EIC) for OXC-201 (€ 2.5MM, 2023-2025) which means that OXC-201 is almost completely financed through preclinical safety assessment studies and the first-in-human, healthy volunteer study. Read more about the TOPFIBRO project here.
“At Oxcia, we are proud to follow in the footsteps of Prof Tomas Lindahl, who won the Nobel Prize for discovering the base excision repair pathway including OGG1. We have developed the first and most advanced OGG1 inhibitor.“
— Ulrika Warpman Berglund, CEO

Key supporting data
OXC-201, has been evaluated in disease models and shown to exhibit a combination of anti-inflammatory and anti-fibrotic effects, inhibiting the production of inflammatory mediators such as TNFα and inhibiting the major hallmarks of fibrogenesis such as TGFβ and collagen1 deposition.
OGG1’s implication in fibrogenesis, combined with its role in inflammation, highlights this enzyme as a promising therapeutic target for ILD, e.g., IPF treatment. Oxcia’s OGG1 inhibitor has preliminary data showing that it protects from acute lung inflammation and pulmonary fibrosis (1). It has furthermore potential to become a promising future treatment of pulmonary fibrosis as a consequence of viral infection (e.g. Covid 19).

Intellectual property
The OGG1 inhibitor is protected by patent in the major countries.
Publications
- Small-molecule-mediated OGG1 inhibition attenuates pulmonary inflammation and lung fibrosis in a murine lung fibrosis model, Lloyd Tanner, Andrew Single, Ravi Kiran Varma Bhongir, Moritz Heusel, Tirthankar Mohanty, Christoffer Karlsson, Lang Pan, Carl-Magnus Clausson, Jesper Bergwik, Ke Wang, Cecilia Andersson, Riya Oommen, Jonas Erjefält, Johan Malmström, Olov Wallner, Istvan Boldogh, Thomas Helleday, Christina Kalderén, Arne Egesten, Nature Comm. 2023; 14: 643
- Optimization of N-Piperidinyl-Benzimidazolone Derivatives as Potent and Selective Inhibitors of 8-Oxo-Guanine DNA Glycosylase 1, Olov Wallner, Armando Cázares-Körner, Emma Rose Scaletti, Geoffrey Masuyer, Tove Bekkhus, Torkild Visnes , Kirill Mamonov, Florian Ortis, Thomas Lundbäck, Maria Volkova, Tobias Koolmeister, Elisée Wiita, Olga Loseva, Monica Pandey, Evert Homan, Carlos Benítez-Buelga, Jonathan Davies, Martin Scobie, Ulrika Warpman Berglund, Christina Kalderén, Pål Stenmark, Thomas Helleday, Maurice Michel, ChemMedChem, 2023 Jan 3;18(1):e202200310, PMID: 36128847
- 8-Oxoguanine targeted by 8-oxoguanine DNA glycosylase 1 (OGG1) is central to fibrogenic gene activation upon lung injury, Lang Pan, Wenjing Hao, Yaoyao Xue, Ke Wang, Xu Zheng, Jixian Luo, Xueqing Ba, Yang Xiang, Xiaoqun Qin, Jesper Bergwik, Lloyd Tanner, Arne Egesten, Allan R. Brasier, Istvan Boldogh, Nucleic Acids Research, 24 Jan 2023, PMID: 3665127
- Pharmacological OGG1 inhibition decreases murine allergic airway inflammation, Lloyd Tanner, Jesper Bergwik, Ravi K. V. Bhongir, Lang Pan, Caijuan Dong, Olov Wallner, Christina Kalderén, Thomas Helleday, Istvan Boldogh, Mikael Adner, Arne Egesten, Frontiers in Pharmacology, 17 October 2022, PMID: 36324676
- TH5487, a small molecule inhibitor of OGG1, attenuates pulmonary fibrosis by NEDD4L-mediated OGG1 degradation, Huayu Ling, Chuge Song , Yaowei Fang, Yu Yin, Zijun Wu, Yahong Wang, Zhiliang Xu, Shenglan Gao, Ao Li , Gang Liu, Chemico-Biological Interactions, Volume 362, 1 August 2022, PMID: 36651270
- Defects in 8-oxo-guanine repair pathway cause high frequency of C > A substitutions in neuroblastoma. , van den Boogaard ML, Oka R, Hakkert A, Schild L, Ebus ME, van Gerven MR, Zwijnenburg DA, Molenaar P, Hoyng LL, Dolman MEM, Essing AHW, Koopmans B, Helleday T, Drost J, van Boxtel R, Versteeg R, Koster J, Molenaar JJ. Proc Natl Acad Sci U S A. 2021 Sep 7;118(36):e2007898118. PMID: 34479993
- Small molecule inhibitor of OGG1 blocks oxidative DNA damage repair at telomeres and potentiates methotrexate anticancer effects. , Baquero JM, Benítez-Buelga C, Rajagopal V, Zhenjun Z, Torres-Ruiz R, Müller S, Hanna BMF, Loseva O, Wallner O, Michel M, Rodríguez-Perales S, Gad H, Visnes T, Helleday T, Benítez J, Osorio A. Sci Rep. 2021 Feb 10;11(1):3490. PMID: 33568707
- NEIL1 and NEIL2 Are Recruited as Potential Backup for OGG1 upon OGG1 Depletion or Inhibition by TH5487. , Hanna BMF, Michel M, Helleday T, Mortusewicz O. Int J Mol Sci. 2021 Apr 27;22(9):4542. PMID: 33925271
- OGG1 Inhibitor TH5487 Alters OGG1 Chromatin Dynamics and Prevents Incisions. , Hanna BMF, Helleday T, Mortusewicz O. Biomolecules. 2020 Oct 26;10(11):1483. PMID: 33114607
- Targeting OGG1 arrests cancer cell proliferation by inducing replication stress. ,Visnes T, Benítez-Buelga C, Cázares-Körner A, Sanjiv K, Hanna BMF, Mortusewicz O, Rajagopal V, Albers JJ, Hagey DW, Bekkhus T, Eshtad S, Baquero JM, Masuyer G, Wallner O, Müller S, Pham T, Göktürk C, Rasti A, Suman S, Torres-Ruiz R, Sarno A, Wiita E, Homan EJ, Karsten S, Marimuthu K, Michel M, Koolmeister T, Scobie M, Loseva O, Almlöf I, Unterlass JE, Pettke A, Boström J, Pandey M, Gad H, Herr P, Jemth AS, El Andaloussi S, Kalderén C, Rodriguez-Perales S, Benítez J, Krokan HE, Altun M, Stenmark P, Berglund UW, Helleday T. Nucleic Acids Res. 2020 Dec 2;48(21):12234-12251. PMID: 33211885
- Computational and Experimental Druggability Assessment of Human DNA Glycosylases. , Michel M, Visnes T, Homan EJ, Seashore-Ludlow B, Hedenström M, Wiita E, Vallin K, Paulin CBJ, Zhang J, Wallner O, Scobie M, Schmidt A, Jenmalm-Jensen A, Warpman Berglund U, Helleday T. ACS Omega. 2019 Jul 5;4(7):11642-11656. PMID: 31460271
- Small-molecule inhibitor of OGG1 suppresses proinflammatory gene expression and inflammation. , Visnes T, Cázares-Körner A, Hao W, Wallner O, Masuyer G, Loseva O, Mortusewicz O, Wiita E, Sarno A, Manoilov A, Astorga-Wells J, Jemth AS, Pan L, Sanjiv K, Karsten S, Gokturk C, Grube M, Homan EJ, Hanna BMF, Paulin CBJ, Pham T, Rasti A, Berglund UW, von Nicolai C, Benitez-Buelga C, Koolmeister T, Ivanic D, Iliev P, Scobie M, Krokan HE, Baranczewski P, Artursson P, Altun M, Jensen AJ, Kalderén C, Ba X, Zubarev RA, Stenmark P, Boldogh I, Helleday T. Science. 2018 Nov 16;362(6416):834-839. PMID: 30442810
- Germline variation in the oxidative DNA repair genes NUDT1 and OGG1 is not associated with hereditary colorectal cancer or polyposis. , Mur P, Jemth AS, Bevc L, Amaral N, Navarro M, Valdés-Mas R, Pons T, Aiza G, Urioste M, Valencia A, Lázaro C, Moreno V, Puente XS, Stenmark P, Warpman-Berglund U, Capellá G, Helleday T, Valle L. Hum Mutat. 2018 Sep;39(9):1214-1225. PMID: 29900613