OXC-101 introduction
OXC-101, a mitotic MTH1 inhibitor, is based on many years research at the Helleday lab at Karolinska Institute and in collaboration with academic groups worldwide such as Mayo clinic, University of Manchester, Uppsala University, Université de Nantes, Shanghai Jiao Tong University, Netherlands Cancer Institute, Sahlgrenska University Hospital, University of Copenhagen, National and Kapodistria University of Athens and Sanger Institute. OXC-101 is investigated in cancer patients with advanced solid cancers and blood cancers.
The problem
Despite targeted medicine and immune-oncology drugs, cancer is still one of the leading causes of death. Globally 19.3 million cancer cases in 2020 and 10 million deaths. There is still an increasing incidence, due to our lifestyle and aging population, which is devastating for patients and places a burden on the healthcare system. Cancers are heterogenous diseases, not only between indications but also within same indication, between patients and within tumor and cancer cells. Furthermore, therapy resistance is a major problem. It is responsible for most relapses of cancer, one of the major causes of death of the disease. Approximately 90% of failures in chemotherapy are related to drug resistance.
Chemotherapy is effective, but in addition to resistance development, severe side-effects is a major concern as it affects both cancer cells and healthy cells. Targeted medicine and immune-oncology drugs have contributed with substantial clinical benefits but unfortunately do not work for all patients. In other words, the medical need is still huge for a safe and effective drug that works in the majority of patients and works in heterogenous and resistant cancers.

The solution
Oxcia is developing OXC-101, a mitotic MTH1 inhibitor, fighting cancer by taking advantage of one of the Achilles heels of cancer cells- the high endogenous oxidative stress and DNA damage. OXC-101 both stops cell division resulting in additional oxidative stress to the cancer cell and ensures that cancer cells cannot repair the oxidized DNA damage. The cancer cell cannot grow and subsequently dies. This dual synergistic mechanism is unique for OXC-101; increasing efficacy, tolerability and size of applicable patient population.
Mode of Action
Background to Mode of Action
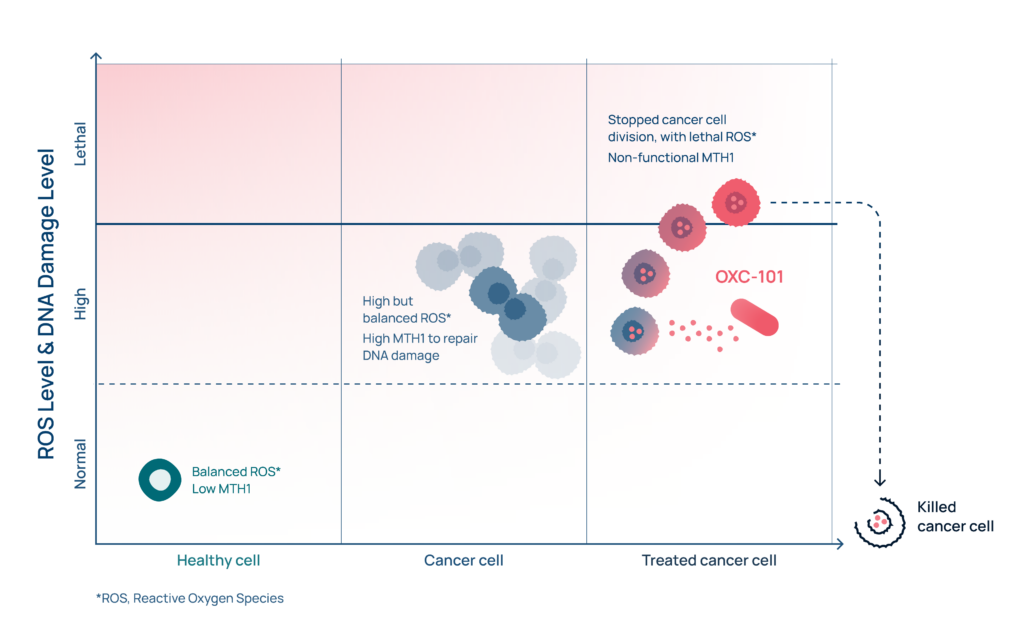
Oxygen is vital for life. In the body’s cells, oxygen can easily form ROS (i.e. Reactive Oxygen Species). This is a normal process in living cells, which is usually highly controlled.
If an imbalance in these control systems occurs, we call it oxidative stress. Then ROS levels can accumulate, and ROS will react with cellular components like DNA and cell membranes and cause damage. This damage can lead to the onset of various diseases like cancer and inflammation.
DNA damage is controlled and repaired in the body by DNA Damage Response (DDR) proteins.
Cancer cells suffers from oxidative stress and elevated levels of ROS and DNA damage.
The DNA damage are usually controlled by production of increased numbers of DDR repair proteins so that oxidative DNA damage is kept below a lethal threshold and the cancer cell is able to survive and multiply.
Unique synergistic dual action makes the difference
OXC-101 is a so-called “mitotic MTH1 inhibitor”. Mitotic MTH1 inhibitors is a completely new class of drugs. No similar compound exists in the pipeline of other companies. OXC-101 is fighting cancer by taking advantage of one of the Achilles heels of cancer cells – the high endogenous oxidative stress and DNA damage. OXC-101 has a unique synergistic dual mechanism of action, stopping cell division and the DNA repair protein MTH1. To facilitate understanding of how it actually works, Oxcia has made an animation which you can find below.
In short, OXC-101 introduces additional oxidative stress and ensures the cancer cells cannot repair the oxidized DNA damage.
In more detail, OXC-101 stops the division of the cancer cell by disturbing microtubule function. This generates additional oxidative stress (i.e. ROS) and increased oxidative DNA building blocks (e.g. 8-oxodGTP). By inhibiting MTH1, a DNA repair protein that cleanses oxygen-damaged DNA building blocks in the cancer cell, more oxidative DNA building blocks are available for incorporation into DNA causing DNA damage. The resulting DNA damage becomes so high that the cancer cell is beyond repair and dies.
Healthy cells are only marginally impacted (as they have limited oxidative DNA damage and therefore no need to repair), which forms the foundation for OXC-101’s excellent tolerability. (For more in depth reading see references 1,2,3)
Notes:
(1) Gad et al Nature 2014 (2) Warpman Berglund et al Annals Oncology 2016 (3) Gad et al BioRxive 2019
Key supporting data
OXC-101 was developed at Karolinska Institutet in Prof. Thomas Helleday’s laboratory. In collaboration with KI and other national and international academic groups, extensive preclinical research has been performed. OXC-101 has been shown to have a broad anti-cancer effect in various disease models, including chemotherapy resistant cancers. This data has been published in several highly-ranked journals. The anticancer activity is equally good or better compared to standard of care in disease models. Therefore OXC-101 has great potential for many cancer indications, cancer types and in many phases of the disease – as a single agent or in combination.It is well tolerated in animals (mice, rat and dog) with no acute toxicity.
OXC-101 alters immune checkpoint markers on cancer cells and recruits cytotoxic T cells to the tumour. Thus, OXC-101 has potential to improve immune-oncology therapy by making cold tumours hot.
Clinical phase I trials in advanced cancer patients also show that OXC-101 is well tolerated. There are preliminary signs of clinical benefits with OXC-101 in these heavily pretreated advanced cancer patients. A recommended phase 2 dose in advanced solid cancers has been established.
Benefits
OXC-101 has the potential to increase survival and has fewer adverse effects as compared to chemotherapy.
In various cancer disease models, OXC-101 has been shown to have potential for a broad anti-cancer effect, can be given both alone and in combination with other approved anticancer treatment and can function in chemotherapy-resistant tumours.
OXC-101 has potential to increase the response of immuno-oncology treatment (making cold tumours hot).
Benefit summary:
Works on several cancer indications ✓
Works on heterogeneous cancers ✓
Healthy cells are not affected ✓
Less general cytotoxic side effects ✓
Potential to overcome existing chemotherapy resistance mechanisms ✓
Mono-, combination-therapy ✓
Oral treatment ✓
Intellectual Property
The patent protection for OXC-101 is strong with large geographical coverage. The major patent covers pyrimidine-2,4-diamine derivatives for treatment of cancer. The patent portfolio also includes patents for other indications and patents for back-up compounds.
Publications
Co-expression of MTH1 and PMS2 is associated with advanced disease and disease progression after therapy in melanoma. , Das I, Tuominen R, Helleday T, Hansson J, Berglund UW, Brage SE. J Invest Dermatol. 2021 Aug 18:S0022-202X(21)01689-4. PMID: 34418425
MTH1 as a target to alleviate T cell driven diseases by selective suppression of activated T cells. , Karsten S, Fiskesund R, Zhang XM, Marttila P, Sanjiv K, Pham T, Rasti A, Bräutigam L, Almlöf I, Marcusson-Ståhl M, Sandman C, Platzack B, Harris RA, Kalderén C, Cederbrant K, Helleday T, Warpman Berglund U. Cell Death Differ. 2021 Aug 27. PMID: 34453118
MTH1 Inhibitors for the Treatment of Psoriasis. , Bivik Eding C, Köhler I, Verma D, Sjögren F, Bamberg C, Karsten S, Pham T, Scobie M, Helleday T, Warpman Berglund U, Enerbäck C. J Invest Dermatol. 2021 Aug;141(8):2037-2048.e4. PMID: 33676948
Karonudib has potent anti-tumor effects in preclinical models of B-cell lymphoma. , Oksvold MP, Berglund UW, Gad H, Bai B, Stokke T, Rein ID, Pham T, Sanjiv K, Øy GF, Norum JH, Smeland EB, Myklebust JH, Helleday T, Våtsveen TK. Sci Rep. 2021 Mar 18;11(1):6317. PMID: 33737576
TH1579, MTH1 inhibitor, delays tumour growth and inhibits metastases development in osteosarcoma model. , Moukengue B, Brown HK, Charrier C, Battaglia S, Baud’huin M, Quillard T, Pham TM, Pateras IS, Gorgoulis VG, Helleday T, Heymann D, Berglund UW, Ory B, Lamoureux F. EBioMedicine. 2020 Mar;53:102704. PMID: 32151797
MTH1 Inhibitor TH588 Disturbs Mitotic Progression and Induces Mitosis-Dependent Accumulation of Genomic 8-oxodG. , Rudd SG, Gad H, Sanjiv K, Amaral N, Hagenkort A, Groth P, Ström CE, Mortusewicz O, Berglund UW, Helleday T. Cancer Res. 2020 Sep 1;80(17):3530-3541. PMID: 32312836
AXL and CAV-1 play a role for MTH1 inhibitor TH1579 sensitivity in cutaneous malignant melanoma. , Das I, Gad H, Bräutigam L, Pudelko L, Tuominen R, Höiom V, Almlöf I, Rajagopal V, Hansson J, Helleday T, Egyházi Brage S, Warpman Berglund U. Cell Death Differ. 2020 Jul;27(7):2081-2098. PMID: 31919461
MutT homologue 1 (MTH1) removes N6-methyl-dATP from the dNTP pool. ,Scaletti ER, Vallin KS, Bräutigam L, Sarno A, Warpman Berglund U, Helleday T, Stenmark P, Jemth AS. J Biol Chem. 2020 Apr 10;295(15):4761-4772. PMID: 32144205
Karonudib is a promising anticancer therapy in hepatocellular carcinoma. , Hua X, Sanjiv K, Gad H, Pham T, Gokturk C, Rasti A, Zhao Z, He K, Feng M, Zang Y, Zhang J, Xia Q, Helleday T, Warpman Berglund U. Ther Adv Med Oncol. 2019 Aug 23;11:1758835919866960. PMID: 31489034
MutT homologue 1 (MTH1) catalyzes the hydrolysis of mutagenic O6-methyl-dGTP. Jemth AS, Gustafsson R, Bräutigam L, Henriksson L, Vallin KSA, Sarno A, Almlöf I, Homan E, Rasti A, Warpman Berglund U, Stenmark P, Helleday T. Nucleic Acids Res. 2018 Nov 16;46(20):10888-10904. PMID: 30304478
Crystal Structures and Inhibitor Interactions of Mouse and Dog MTH1 Reveal Species-Specific Differences in Affinity. , Narwal M, Jemth AS, Gustafsson R, Almlöf I, Warpman Berglund U, Helleday T, Stenmark P. Biochemistry. 2018 Feb 6;57(5):593-603. PMID: 29281266
A patient-derived xenograft pre-clinical trial reveals treatment responses and a resistance mechanism to karonudib in metastatic melanoma. , Einarsdottir BO, Karlsson J, Söderberg EMV, Lindberg MF, Funck-Brentano E, Jespersen H, Brynjolfsson SF, Olofsson Bagge R, Carstam L, Scobie M, Koolmeister T, Wallner O, Stierner U, Berglund UW, Ny L, Nilsson LM, Larsson E, Helleday T, Nilsson JA. Cell Death Dis. 2018 Jul 24;9(8):810. PMID: 30042422
Germline variation in the oxidative DNA repair genes NUDT1 and OGG1 is not associated with hereditary colorectal cancer or polyposis. , Mur P, Jemth AS, Bevc L, Amaral N, Navarro M, Valdés-Mas R, Pons T, Aiza G, Urioste M, Valencia A, Lázaro C, Moreno V, Puente XS, Stenmark P, Warpman-Berglund U, Capellá G, Helleday T, Valle L. Hum Mutat. 2018 Sep;39(9):1214-1225. PMID: 29900613
Fragment-Based Discovery and Optimization of Enzyme Inhibitors by Docking of Commercial Chemical Space. , Rudling A, Gustafsson R, Almlöf I, Homan E, Scobie M, Warpman Berglund U, Helleday T, Stenmark P, Carlsson J. J Med Chem. 2017 Oct 12;60(19):8160-8169. PMID: 28929756
Glioblastoma and glioblastoma stem cells are dependent on functional MTH1. , Pudelko L, Rouhi P, Sanjiv K, Gad H, Kalderén C, Höglund A, Squatrito M, Schuhmacher AJ, Edwards S, Hägerstrand D, Berglund UW, Helleday T, Bräutigam L. Oncotarget. 2017 Jul 20;8(49):84671-84684. PMID: 29156675
Validation and development of MTH1 inhibitors for treatment of cancer., Warpman Berglund U, Sanjiv K, Gad H, Kalderén C, Koolmeister T, Pham T, Gokturk C, Jafari R, Maddalo G, Seashore-Ludlow B, Chernobrovkin A, Manoilov A, Pateras IS, Rasti A, Jemth AS, Almlöf I, Loseva O, Visnes T, Einarsdottir BO, Gaugaz FZ, Saleh A, Platzack B, Wallner OA, Vallin KS, Henriksson M, Wakchaure P, Borhade S, Herr P, Kallberg Y, Baranczewski P, Homan EJ, Wiita E, Nagpal V, Meijer T, Schipper N, Rudd SG, Bräutigam L, Lindqvist A, Filppula A, Lee TC, Artursson P, Nilsson JA, Gorgoulis VG, Lehtiö J, Zubarev RA, Scobie M, Helleday T. Ann Oncol. 2016 Dec;27(12):2275-2283. PMID: 27827301
Hypoxic Signaling and the Cellular Redox Tumor Environment Determine Sensitivity to MTH1 Inhibition. , Bräutigam L, Pudelko L, Jemth AS, Gad H, Narwal M, Gustafsson R, Karsten S, Carreras Puigvert J, Homan E, Berndt C, Berglund UW, Stenmark P, Helleday T. Cancer Res. 2016 Apr 15;76(8):2366-75. PMID: 26862114
hMYH and hMTH1 cooperate for survival in mismatch repair defective T-cell acute lymphoblastic leukemia. , Eshtad S, Mavajian Z, Rudd SG, Visnes T, Boström J, Altun M, Helleday T. Oncogenesis. 2016 Dec 5;5(12):e275. PMID: 27918552
Pathways controlling dNTP pools to maintain genome stability. , Rudd SG, Valerie NCK, Helleday T. DNA Repair (Amst). 2016 Aug;44:193-204. PMID: 27311542 Review.
Crystal structure, biochemical and cellular activities demonstrate separate functions of MTH1 and MTH2. , Carter M, Jemth AS, Hagenkort A, Page BD, Gustafsson R, Griese JJ, Gad H, Valerie NC, Desroses M, Boström J, Warpman Berglund U, Helleday T, Stenmark P. Nat Commun. 2015 Aug 4;6:7871. PMID: 26238318
Addiction to MTH1 protein results in intense expression in human breast cancer tissue as measured by liquid chromatography-isotope-dilution tandem mass spectrometry. , Coskun E, Jaruga P, Jemth AS, Loseva O, Scanlan LD, Tona A, Lowenthal MS, Helleday T, Dizdaroglu M. DNA Repair (Amst). 2015 Sep;33:101-10. PMID: 26202347
Development and validation of method for TH588 and TH287, potent MTH1 inhibitors and new anti-cancer agents, for pharmacokinetic studies in mice plasma. , Saleh A, Gökturk C, Warpman-Berglund U, Helleday T, Granelli I. J Pharm Biomed Anal. 2015 Feb;104:1-11. PMID: 25459754
Stereospecific targeting of MTH1 by (S)-crizotinib as an anticancer strategy. , Huber KV, Salah E, Radic B, Gridling M, Elkins JM, Stukalov A, Jemth AS, Göktürk C, Sanjiv K, Strömberg K, Pham T, Berglund UW, Colinge J, Bennett KL, Loizou JI, Helleday T, Knapp S, Superti-Furga G. Nature. 2014 Apr 10;508(7495):222-7. PMID: 24695225
MTH1 inhibition eradicates cancer by preventing sanitation of the dNTP pool. , Gad H, Koolmeister T, Jemth AS, Eshtad S, Jacques SA, Ström CE, Svensson LM, Schultz N, Lundbäck T, Einarsdottir BO, Saleh A, Göktürk C, Baranczewski P, Svensson R, Berntsson RP, Gustafsson R, Strömberg K, Sanjiv K, Jacques-Cordonnier MC, Desroses M, Gustavsson AL, Olofsson R, Johansson F, Homan EJ, Loseva O, Bräutigam L, Johansson L, Höglund A, Hagenkort A, Pham T, Altun M, Gaugaz FZ, Vikingsson S, Evers B, Henriksson M, Vallin KS, Wallner OA, Hammarström LG, Wiita E, Almlöf I, Kalderén C, Axelsson H, Djureinovic T, Puigvert JC, Häggblad M, Jeppsson F, Martens U, Lundin C, Lundgren B, Granelli I, Jensen AJ, Artursson P, Nilsson JA, Stenmark P, Scobie M, Berglund UW, Helleday T. Nature. 2014 Apr 10;508(7495):215-21PMID: 24695224
Cancer phenotypic lethality, exemplified by the non-essential MTH1 enzyme being required for cancer survival. , Helleday T. Ann Oncol. 2014 Jul;25(7):1253-1255. PMID: 24737777 Crystal structure of human MTH1 and the 8-oxo-dGMP product complex. , Svensson LM, Jemth AS, Desroses M, Loseva O, Helleday T, Högbom M, Stenmark P. FEBS Lett. 2011 Aug 19;585(16):2617-21. PMID: 21787772